
High-level international conference on
African development, initiated by Japan
The government of Japan launched the Tokyo International Conference on
African Development (TICAD) in 1993, co-hosting this high-level summit with the United Nations,
United Nations Development Programme (UNDP), World Bank, and African Union Commission (AUC)
since that time. Based Japan’ foreign policy priorities—namely human security—TICAD plays an
important role in informing and strengthening Japan’s notable partnerships with African
countries, providing a platform for critical global collaboration in support of African
sustainable, stable economic growth and development.
Following the success of the 2016 TICAD VI’s convening in Nairobi, Kenya for the first time in
Africa, TICAD will take place every three years, alternating between Japan and Africa. TICAD VII
will be held from August 28 to 30, 2019 at Pacifico Yokohama, Yokohama city, Japan, with the
theme: “Advancing African Development through People, Technology and Innovation.”
GHIT launched at TICAD V (2013)
in Yokohama
Following its launch at TICAD V, GHIT has facilitated open innovation for global health between Japanese and non-Japanese entities. GHIT’s side event at TICAD VI in Nairobi, Kenya in August 2016 welcomed former Japanese Health Minister Hon. Mr. Yasuhisa Shiozaki, Hideyo Noguchi Prize Laureates, and global health leaders to highlight R&D partnerships between Japan and Africa.
TICAD V GHIT side event reportTICAD VI GHIT side event report
The GHIT portfolio’s most advanced clinical candidates in Africa
The three most advanced clinical candidates in GHIT’s portfolio include tuberculosis diagnostics, a new pediatric treatment for schistosomiasis, and a drug for mycetoma. Japan’s innovation plays a critical role in the development of these life-saving, innovative tools to fight neglected diseases in Africa. These clinical trials have been conducted in partnership with African universities and research institutions, as well as development partners around the globe.
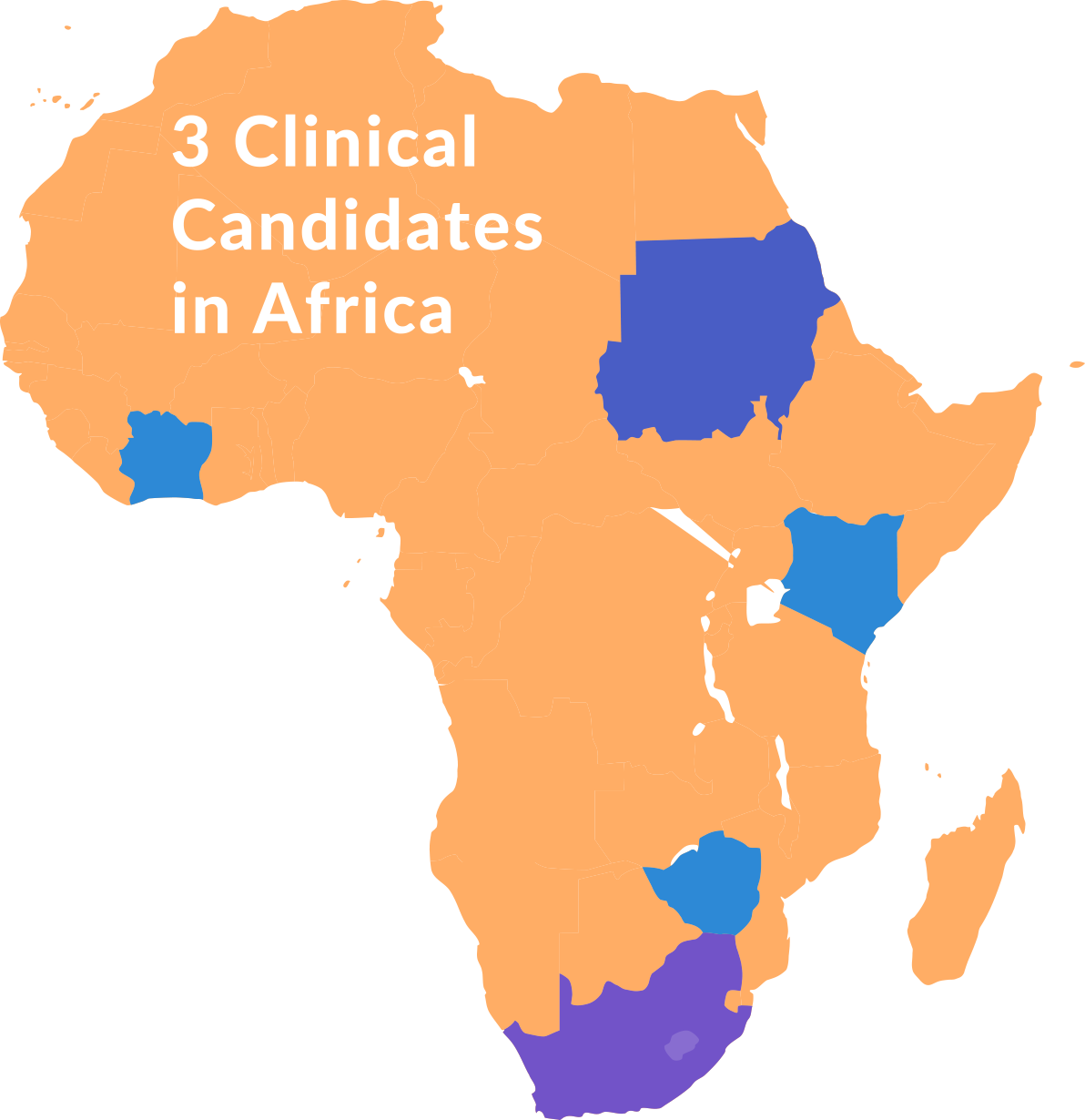
- Sudan
- Cote d’Ivoire
- Kenya
- Zimbabwe
- South Africa
Clinical Candidate 1
SILVAMP™ TB-LAM
- Disease: Tuberculosis
- Intervention: Diagnostics
- Development Stage: Product validation
- Country: South Africa
Novel, urine-based, point-of-care test for TB diagnosis for people living with HIV in low-resource settings
- Challenge
- Tuberculosis (TB) is leading cause of death in HIV Patients. In order to provide appropriate care and treatment to HIV patients, early diagnosis is essential. However, advanced HIV-positive patients presenting for TB diagnosis are unable to produce a sputum sample and many patients cannot be diagnosed in time, which results in high morbidity and high mortality. The world needs a non-sputum based, rapid diagnostic tool that can be used in LMICs with resource limited settings.
- Innovation
- SILVAMP™ TB-LAM uses patients’ urine to diagnose TB, detecting low concentrations of LAM (lipoarabinomannan), which is found in the cell walls of mycobacterium tuberculosis. The partnership successfully leverages FUJIFILM’s proprietary silver halide amplification technology, originally developed for processing photographs, to create highly sensitive immunochromatography, which is capable of detecting viruses and bacteria.
Product Development Partners

Clinical Candidate 2
Pediatric Praziquantel (PZQ)
- Disease: Schistosomiasis
- Intervention: Pediatric Drug
- Development Stage: Phase III
- Country: Cote d’Ivoire, Kenya, and Zimbabwe
A new pediatric formulation of the gold-standard drug -- smaller in size, less bitter, and orally dispersible
- Challenge
- Schistosomiasis is the second-most socioeconomically devastating parasitic disease after malaria. Exposure leaves infected children vulnerable to increased risk for anaemia, stunting, and a reduced ability to learn, although the effects are usually reversible with treatment. Praziquantel (PZQ), an affordable, gold-standard drug, exists, but treatment for preschool-aged children has not been implemented due to several key obstacles: difficulty in swallowing and risk of choking give the large-sized commercially available tablets and high rejection rates due to bitter taste when pills are crushed and dispersed in liquids.
- Innovation
- By utilizing Astellas’ formulation technology, the new pediatric tablets are about a quarter of the size of the existing commercial PZQ tablets and are orally dispersible (can be taken with or without water). This, together with agents that minimize PZQ’s bitterness and improve the taste of the tablets, helps children swallow more easily, which facilitates compliance and efficacy. In addition, the tablets are heat-stable in hot and humid African environments.
Product Development Partners

Clinical Candidate 3
First-ever clinical trial for potential new effective, lower cost mycetoma drug
- Challenge
- Mycetoma (Eumycetoma), caused by a fungal infection, slowly and progressively destroys soft tissue, particularly on the feet. Mycetoma has no effective treatment and is currently managed with sub-optimal, expensive drugs carrying strong side effects and often requiring surgery, including amputation of affected limbs. In rare cases, it affects the lungs or brain and can be fatal. In all cases, patients are unable to work and face severe social stigma.
- Innovation
- Fosravuconazole, originally developed by Eisai to treat other fungal infections, has a proven safety profile and shows strong in vitro antifungal activity against mycetoma. This candidate drug can be administered once weekly and would drastically improve patient compliance and reduce costs for individuals and health systems alike. This drug, if successful, could transform their quality of life.
Product Development Partners

TICAD VII
Side Event2019.8.28
Official side events hosted by GHIT will take place on August 28.
“Advancing African Development through People, Technology and Innovation.”
GHIT is co-organizing two events on the sidelines of TICAD VII, both taking place on August
28.
Invited speakers, partners, and attendees include African Heads of State, high-officials of the
Government of Japan,
and representatives from private companies, universities, research organizations, international
organizations, and NGO/NPOs.
The events will explore the legacy of Japan-African partnerships and initiatives in the fight
against neglected diseases,
highlight African countries’ success stories and Japan's innovation and technology, and identify
access and
delivery efforts contributing to disease elimination and the attainment of universal health
coverage.
We look forward to your participation and engagement and see you in Yokohama!
-
Partnering for Progress:
Promoting Innovation & Access for
Neglected Diseases in furtherance of UHC -
REGISTER
Organizer
United Nations Development Program (UNDP)/ Access and Delivery Partnership (ADP)
United Nations Volunteers (UNV) programme
GHIT Fund
- An Africa free from Neglected Tropical Diseases (NTDs) - A partnership between Africa and Japan
-
REGISTER
Organizer
Japan Alliance on Global Neglected Tropical Diseases (JAGntd)
GHIT Fund
Co-organizer
DNDi Japan
Eisai Co., Ltd.
Japan International Cooperation Agency (JICA)
Japan Pharmaceutical Manufacturers Association (JPMA)
SDGs Promise Japan
Uniting to Combat NTDs (UTC)
Advancing African Development
through People,
Technology and Innovation.



