GHIT 3.0
Strategic Plan
FY2023-FY2027
- Since its inception in 2013, the GHIT Fund has been investing in new product development to contribute to global health, supporting Japanese technology and innovation to combat infectious diseases, such as malaria, tuberculosis, and neglected tropical diseases (NTDs), which affect the world's vulnerable and underserved populations. "GHIT 3.0," the third five-year plan which began in April 2023 after the 10th anniversary of the GHIT Fund's founding, sets the following three pillars of its strategy, aiming to streamline and accelerate R&D and bring products to patients as quickly as possible.
-

PDF Download
Galvanize Innovation
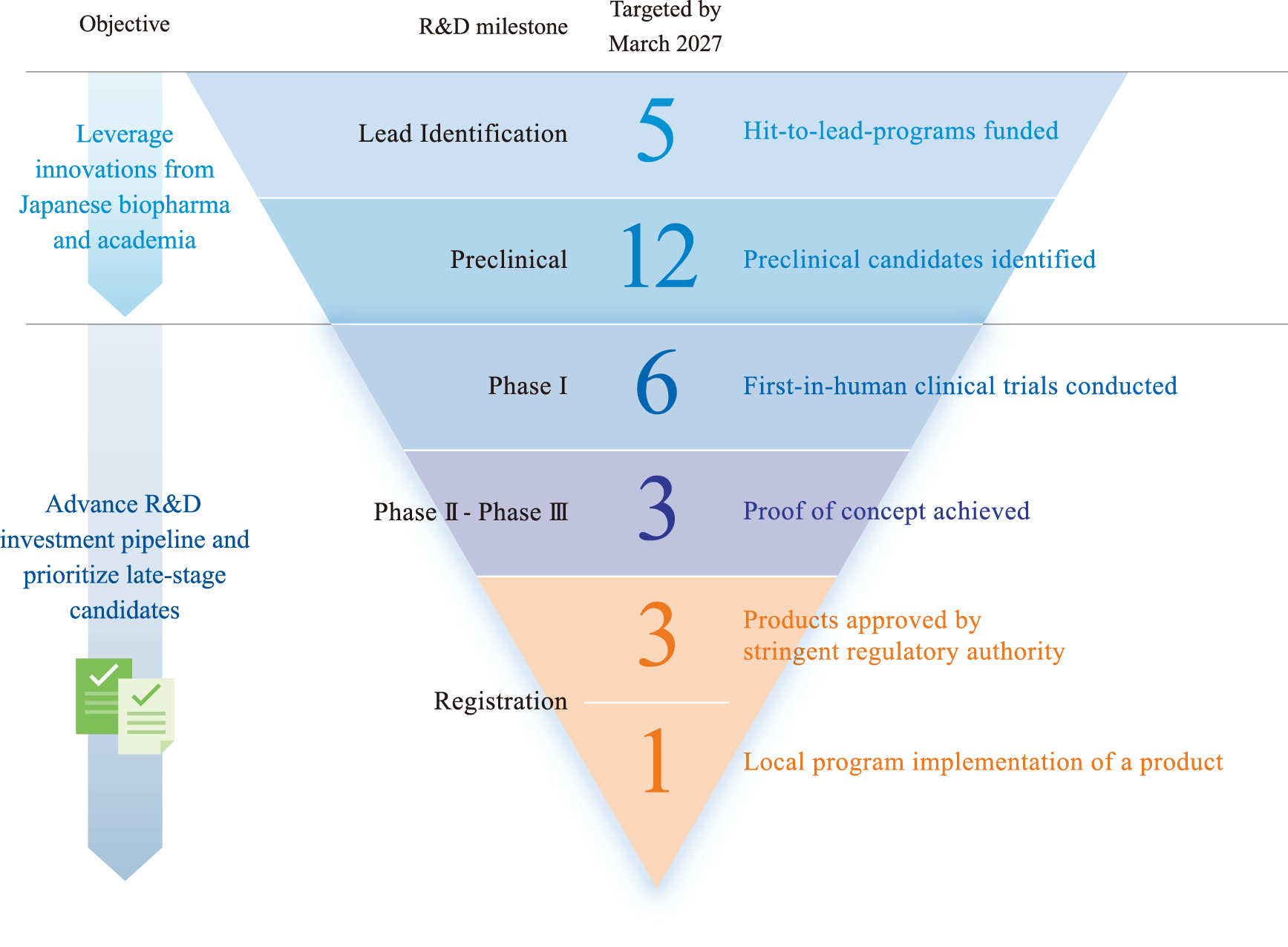
- Continue to invest in product development to invigorate innovation in R&D by Japanese and global stakeholders in each stage of R&D, from discovery to clinical to regulatory approval phases.
- Actively invest with prioritization of late-stage product development programs to pave the way for faster delivery of drugs, vaccines and diagnostics to the people who need them the most.
- Promote the co-creation of products through exploration of innovative technology and promote global, cross-sectoral collaborations across academia, research institutions, pharmaceutical companies, small and medium-sized enterprises and start-up companies, that span multiple disciplines such as pandemic preparedness and climate change.
Maximize Impact
- Accelerate product development for tuberculosis, malaria and NTDs and optimize portfolio and resources to maximize investment impact.
- Explore various funding mechanisms to continue sustainable and efficient funding for R&D using limited resources (e.g., funding, personnel, etc).
- Enhance institutional development, for example, through reinforcement of compliance and risk management and promotion of greater transparency of decision-making processes.
Optimize Portfolio
- GHIT 1.0
- FY2013-FY2017

- Phase I: Creation
-
- Establish an innovative funding mechanism to create international public goods from Japan through public-private partnership (Japanese government x private companies x foundations).
- Launch R&D projects utilizing Japanese drug discovery technologies and innovations.
- GHIT 2.0
- FY2018-FY2022

- Phase II: Growth
-
- Advance product development pipelines through active management of existing portfolio alongside proactive new investments in innovative technologies applicable to global health.
- Support access and delivery through close collaboration with international organizations.
- GHIT 3.0
- FY2023-FY2027

- Phase III: Harvest
-
- Accelerate product development for malaria, tuberculosis and NTDs.
- Optimize portfolio and resources to maximize the investment impact and further strengthen institutional development.
- Foster and promote domestic and international partnerships aimed at sparking innovation.
Leverage Resources
-
- Continue to leverage limited resources by exploring various funding mechanisms and prioritizing sustainability.
Enhance Governance and Institutional Management
-
- Further enhance corporate governance and institutional development.
- Ensure organizational sustainability through deployment of appropriate HR management and financial strategies.
Catalyze Partnerships
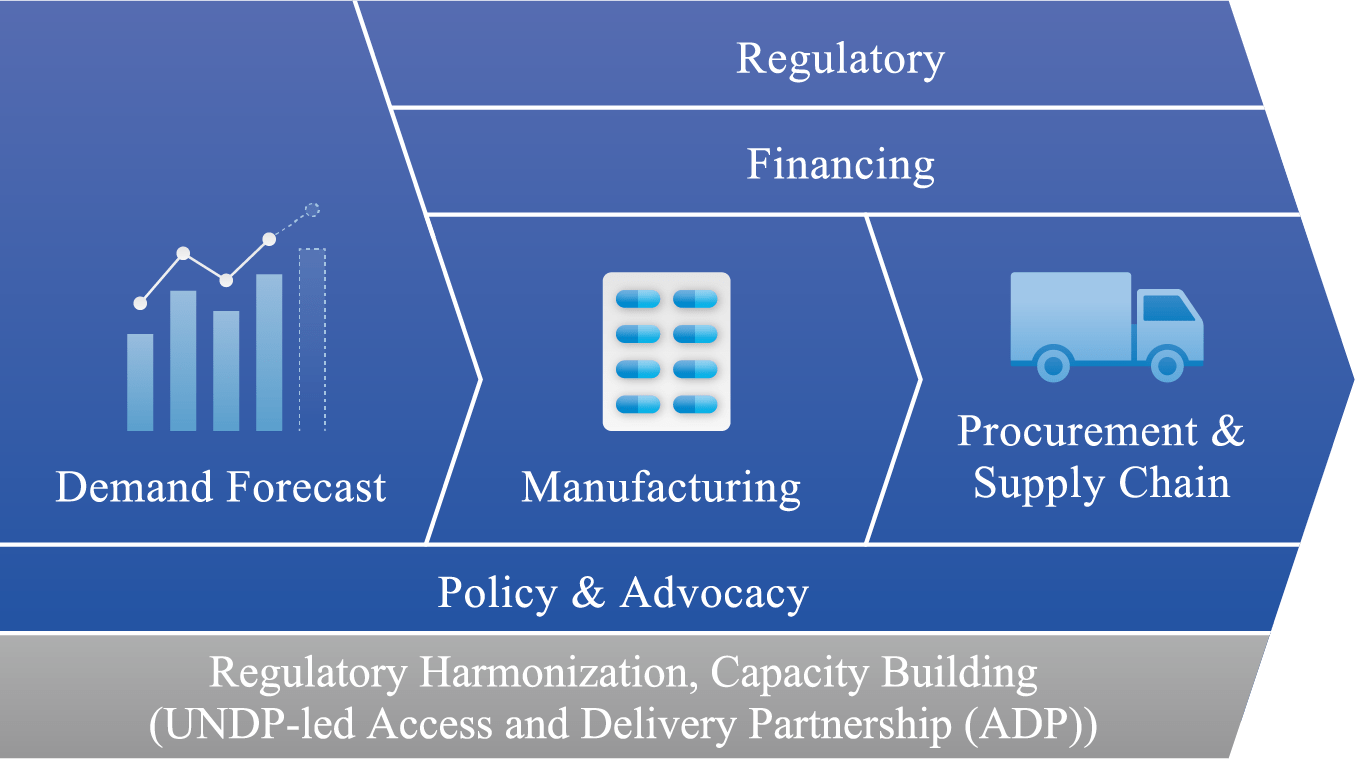
Proactively collaborate with product development partners to help them develop robust launch strategies and establish strategic, product-focused partnerships to create an environment for effective access and delivery to deliver drugs, vaccines and diagnostics to the field more quickly.
Create an Enabling Environment for Access& Delivery
Access & Delivery
Patient

- GHIT facilitates strategic partnerships for Access and Delivery

- Top-down approach
- Create a platform for aligned action across the innovation-access-delivery continuum.

- Bottom-up approach
- Ensure launch strategies for GHIT-invested clinical candidates
Strengthen Strategic Partnerships, Focusing on LICs & LMICs
-
- Continue to extend institutional presence in LICs and LMICs and explore innovationsfrom the ground up.
Demonstrate End-to-End R&D/A&D Ecosystems
-
- Leverage UNDP and other global networks to ensure seamless connectivity between innovation (R&D) and access and delivery(A&D).