-
Project IDT2021-256
-
RFP Year2021
-
Awarded Amount$698,170DiseaseMalariaInterventionVaccineDevelopment StageAntigen IdentificationCollaboration PartnersHokkaido University , Jichi Medical University , Kyoto University , Toyama University , University of Cambridge , Instituto Leônidas & Maria Deane (ILMD) and The Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD) , Kanazawa University
Introduction and Background of the Project
1. Introduction
Malaria has had a profound effect on human lives for thousands of years and remains one of the most serious, life-threatening infectious diseases. Despite past and ongoing efforts to control and reduce mortality and morbidity caused by this disease, it was estimated that annually 241 million people were infected, and 627,000 died in 2020. To exacerbate this situation, the COVID-19 pandemic has further disrupted ongoing malaria services, leading to a marked increase in cases and deaths.
P. falciparum and P. vivax are the two most important human malaria species. Development of malaria vaccines, which has absorbed a large proportion of malaria research funds in recent decades, has concentrated almost exclusively on P. falciparum. Hence, control efforts have focused on reducing the morbidity and mortality associated with falciparum malaria. However, with improved malaria diagnostics, globalization and mutated vivax parasites, there is more evidence of high vivax burden in Africa. In addition, climate change is widely considered to drive the spread of vivax in the immediate future. Despite the fact that P. vivax is more geographically dispersed than P. falciparum, with transmission occurring over a wider range of temperatures than for P. falciparum, P. vivax vaccine development is resolutely still in early preclinical development. In P. falciparum and P. vivax co-endemic areas, an ideal malaria vaccine should be highly efficacious for both parasites.
2. Project objective
Our aim is to develop not only a highly effective and durable multistage vaccine against pre-erythrocytic and sexual stages of P. vivax, but additionally, a bivalent vaccine effective both for P. vivax and P. falciparum.
3. Project design
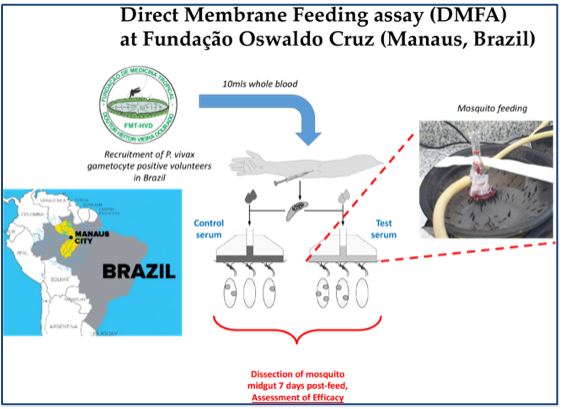
Two viral-vectored vaccines expressing both pre-erythrocytic-stage and sexual-stage antigens of P. vivax will be generated. Protective and transmission blocking (TB) efficacies of the heterologous prime-boost immunization regimen will be assessed by sporozoite challenge and Direct Membrane Feeding Assay (DMFA) in a robust and proven mouse model, and then the regime will be further optimized (e.g., dose, route, interval and outbred mice). Desired protection rate >90%. Surrogate markers responsible for protection will be identified. This will be key to allow efficient and robust measurements of efficacy. Humoral and cellular immune responses induced by the heterologous prime-boost immunization regimen will be assessed. Meanwhile, a bivalent vaccine harboring the genes encoding antigens of both P. vivax and P. falciparum will be generated. In a non-human primate model, in vitro sporozoite neutralizing assay and in vivo sporozoite challenge test of mice passively transferred with immune monkey IgGs will be performed to evaluate its protective efficacy. For evaluation of transmission blocking efficacy, immune monkey sera will be tested by DMFA using blood of vivax patients in Brazil and of falciparum patients in Burkina Faso.
How can your partnership (project) address global health challenges?
The recent (October 2021) endorsement of RTS,S/AS01 for broad use in the field is positive news, and is the first anti-malarial vaccine candidate (and the first vaccine to address parasitic infection) to achieve this key approval milestone. From preliminary data, the inclusion of RTS,S with currently used chemopreventative treatment results in significantly increased efficacy against clinical malaria, hospital admission with severe malaria and mortality. These findings give a clear indication as to the wide and undeniable benefits of incorporating RTS,S within currently existing anti-malarial control measures. Conversely, it is also widely predicted that the incorporation of RTS,S alone into current control measures will not achieve our previously stated aims against malaria, namely long-term control or elimination, because of limited and waning efficacy. Our project aims to develop “second-generation” anti-malarial vaccines, with enhanced characteristics compared with other options, is key for long-term anti-malarial control.
What sort of innovation are you bringing in your project?
To achieve the pressing demand for high, long-lasting malaria vaccine efficacy, we are developing a highly effective and durable next-generation multistage/multispeices malaria vaccine that is effective against both pre-erythrocytic stage and sexual-stage P. falciparum and P. vivax parasites. Our multistage vaccine effective both for protection and transmission has multiple advantages to overcome the potential risks of reducing vaccine effectiveness over time because of sequential gene mutation, polymorphism and subsequent pathogen escape. In addition, the simple two-dose immunization regimen would ideally be tailored for integration into the current Expanded Programme on Immunization (EPI) vaccines for infants.
Role and Responsibility of Each Partner
(1.) Kanazawa university, Japan: Overall project management and proposal design, production of viral-vectored vaccine platform, immunization studies, immunological assays, challenge tests against sporozoites. Kanazawa University has a sophisticated facility capable of evaluating pre-erythrocytic-stage vaccines using sporozoite-infected mosquitoes.
(2.) Jichi Medical University, Japan: Proposal design/consultation and production of viral-vectored vaccine platform.
(3.) Hokkaido University, Japan: Proposal design/consultation and production of viral-vectored vaccine platform.
(4.) Kyoto University, Japan: Proposal design. Rhesus monkey tests are being planned to evaluate its safety, immunogenicity and vaccine efficacy. Immunization of monkeys will conduct safety, immunogenicity and vaccine efficacy test in a monkey model.
(5.) Toyama University, Japan: Proposal design, immunological assays
(6.) University of Cambridge, UK: Proposal design, mouse immunization studies, DMFA conducted in Burkina Faso (in collaboration with IRSS), whom routinely perform these experiments. These results, obtained in a field, “human-only” assay, will facilitate future translation to further human trials. University of Cambridge has a sophisticated facility capable of evaluating TB vaccines using P. falciparum gametocyte culture and field blood samples from malaria patients.
(7.) Instituto Leônidas & Maria Deane (ILMD) and The Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD), Brazil: Proposal design, immunization studies, Evaluation of directly block transmission of P. vivax (field) samples to mosquitoes within a malaria-endemic setting, performed in Manaus. ILMD/Fiocruz Amazônia is the technical-scientific unit of the Oswaldo Cruz Foundation in Amazonas. Headquartered in Manaus, its mission is to contribute to improving the living and health conditions of the Amazon populations and to the regional and national scientific development and public health actions. FMT-HVD, located in Manaus, is a pioneering institution in this region regarding the syndromic surveillance of P. vivax infections. FMT-HVD has played a key role in performing diagnosis and treatment of vivax patients in the Amazon endemic areas.
All partners will be co-responsible for data interpretation.
Others (including references if necessary)
1. WHO. World Malaria Report 2021. (2021).
2. WHO. More malaria cases and deaths in 2020 linked to COVID-19 disruptions (https://www.who.int/news/item/06-12-2021-more-malaria-cases-and-deaths-in-2020-linked-to-covid-19-disruptions). (2021).
3. Moorthy, V. S., Newman, R. D. & Okwo-Bele, J. M. Malaria vaccine technology roadmap. Lancet 382, 1700-1701, doi:10.1016/S0140-6736(13)62238-2 (2013).
4. Sherrard-Smith, E. et al. Synergy in anti-malarial pre-erythrocytic and transmission-blocking antibodies is achieved by reducing parasite density. Elife 7, doi:10.7554/eLife.35213 (2018).
5. Blagborough, A. M. et al. Transmission-blocking interventions eliminate malaria from laboratory populations. Nat Commun 4, 1812, doi:10.1038/ncomms2840 (2013).
Final Report
1. Project objective
Our aim is to develop not only a highly effective and durable next-generation multistage malaria vaccine effective against both pre-erythrocytic stage and sexual-stage P. vivax parasites based on a highly attenuated vaccinia strain; LC16m8 (m8) and adeno-associated virus (AAV), but additionally, a bivalent vaccine effective both for P. vivax and P. falciparum.
2. Project design
The vaccine is based on two viral-vectored vaccines, consisting of m8 and AAV expressing P. vivax Pvs25-PvCSP fusion protein. Heterologous m8-prime/AAV-boost immunization regimen will be performed in animal models. Immunized mice will be challenged against transgenic “humanized” P. berghei parasites expressing PvCSP. In a non-human primate model, immune monkey sera will be evaluated for the safety, immunogenicity and vaccine efficacy by in vitro sporozoite neutralizing assay. For evaluation of transmission blocking efficacy, mouse and monkey immune sera will be tested by membrane feeding assay using blood of vivax patients in Brazil.
3. Results, lessons learned
The m8/AAV Pv vaccine was constructed. For protective efficacy in a mouse model, the heterologous m8-prime/AAV-boost immunization regimen provided 100% (short-term; Day 28) and 60% (long-term; Day 242) protection against PvCSP VK210 transgenic P. berghei sporozoites. For TB efficacy, mouse sera immunized with the vaccine formulation showed >75% TB activity and >95% transmission reduction activity by a direct membrane feeding assay using P. vivax isolates in blood from an infected patient from the Brazilian Amazon region.
For a bivalent vaccine, a m8 vaccine expresses Pfs25-PfCSP and Pvs25-PvCSP fusion proteins, while the AAV1 vaccine includes two recombinant AAV1s carrying one of these cassettes as a mixture. the heterologous m8 /AAV immunization regimen provided 70% protection against both PfCSP/Pb and PvCSP/Pb transgenic sporozoites. Moreover, a direct membrane feeding assay (DMFA) using blood from P. vivax patients in the Brazilian Amazon showed 90% transmission-blocking efficacy. The bivalent vaccine outperformed monovalent combinations, maintaining immune responses for over 7 months, and shows promise for malaria control and elimination.
In a non-human primate model, we confirmed that the m8/AAV Pv vaccine is safe, with acceptable reactogenicity and no detectable systemic toxicity. The vaccine persists high level of anti-PvCSP and Pvs25 antibody titers over a minimum of 6 months. Regarding vaccine efficacy, the monkey immune sera possessed not only high levels of in vitro sporozoite neutralizing activity (92-99%) but also robust levels of transmission blocking efficacy (>99%) . Additionally, the immune monkey sera possess strong neutralizing activity against Mpox, indicating another advantage of the m8/AAV vaccine platform.
Thus, our project demonstrates the feasibility and versatility of this vaccine platform as a P. vivax and Pf-Pv bivalent multistage vaccine in animal models. This vaccine significantly advances malaria elimination strategies by offering durable immunity, cross-species protection, and potent transmission-blocking efficacy. Future research should focus on human clinical trials to evaluate safety, efficacy, and TBA These efforts will be instrumental in developing a next-generation malaria vaccine capable of addressing the global malaria burden.
Investment
Details
Development of a Plasmodium vivax multistage vaccine effective both for protection and transmission blocking




