-
Project IDG2020-203
-
RFP Year2020
-
Awarded Amount$1,471,273DiseaseNTD(Chagas disease)InterventionDiagnosticDevelopment StageProduct ValidationCollaboration PartnersEiken Chemical Co., Ltd. , Nagasaki University , Fundacion Mundo Sano , Ciencia y Estudios Aplicados Para el Desarrollo en Salud y Medio Ambiente (CEADES) , Centro para el Desarrollo de Investigación Científica (CEDIC) , Consejo Nacional de Investigaciones Científicas y Técnicas - Instituto de Investigaciones en Ingeniería Genética y Biología Molecular “Dr. Héctor N. Torres” (CONICET-INGEBI) , AI Biosciences Inc. , Barcelona Institute for Global Health (ISGLOBAL)Publication
Introduction and Background of the Project
Introduction
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, affects ~7 million people, mostly in Latin America. Vector-borne transmission is on the way of being controlled in several countries, but vertical transmission remains an uncontrolled major public-health challenge. Remarkably, available drugs have a very high cure rate in T. cruzi-infected newborns if administered early upon infection, thus a timely diagnose is crucial for treatment success. However, the algorithm to detect congenital T. cruzi infection involves parasitological methods that lack sensitivity and a serological study must be performed several months later. In many endemic regions people live far from referral centers which entails that a large proportion of infants rarely go back for diagnosis confirmation and treatment, if infected. Consequently, they evolve to the chronic phase of the disease with the risk of developing severe manifestations.
Molecular-based diagnostics have a very high sensitivity to detect congenital T. cruzi infections, but laboratories in those distant regions are not equipped to perform them. With the aim to provide a suitable point-of-care (POC) test for the timely diagnosis of congenital Chagas disease in these settings, we will validate the implementation of EIKEN T. cruzi loop-mediated isothermal amplification (LAMP) prototype in the field.
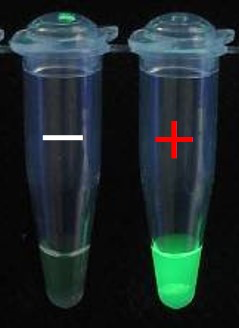
Project objective
Objectives of this project are to: 1) conduct an analytical evaluation of the most suitable DNA isolation methodology for implementing T. cruzi-LAMP as POC test for timely diagnosis of congenitally acquired T. cruzi infection; 2) evaluate operationally its use in maternity hospitals from Chagas disease endemic regions of Argentina, Bolivia and Paraguay; 3) validate in a wide geographical area the use of rapid diagnostic tests (RDTs) as an alternative to conventional enzyme-linked immunosorbent assays (ELISAs) to detect chronic T. cruzi infections; and 4) perform information, education and communication (IEC) and advocacy activities to increase disease awareness and facilitate the potential acceptance and adoptability of the technologies under research by the communities and authorities where the project will be developed.
Project design
We will work in hospitals with maternity wards in Argentina, Bolivia and Paraguay. Infants enrolled will follow current algorithm to diagnose congenital Chagas comprising two microscopy-based observations of parasite presence in peripheral blood at birth and a few months later, and a serological study by nine months of age. Whole blood samples will be obtained at those time-points and stored until needed. In parallel to the recruitment, we will determine the best conditions for implementing EIKEN T. cruzi-LAMP prototype as POC diagnostic, including the evaluation of two techniques to provide the required purified DNA for the reaction: AI Biosciences low-cost 3D printer-inspired platform and EIKEN PURE (Procedure for Ultra Rapid Extraction) system. The most suitable for field use accompanying LAMP will be chosen, bearing in mind their performance and operational parameters like ease of use.
As part of the project, we will also compare the performance of RDTs for the detection of chronic T. cruzi-infected mothers with that of ELISAs. Rationale for the use of RDTs stands on our previous experience in the Bolivian Chaco, where they proved to be highly sensitive and their quick results turnaround allowed swift recruitment of study participants.
According to Chagas disease prevalence reports in the study regions, and an average vertical transmission rate of ~5%, we will need to screen ~16,000 women (mean prevalence of chronic Chagas disease 17.2%) so as to enroll the newborns of those T. cruzi-infected (~2,800). During the second year we will follow the infants and run the T. cruzi-LAMP tests. In addition to current congenital Chagas algorithm, their performance will be compared to that of a standard qPCR molecular-based technique.
How can your partnership (project) address global health challenges?
Chagas disease persists as a major public health challenge. It is endemic to 21 countries in Latin America and causes a greater burden of disability-adjusted life years than any other parasitic disease in the Americas. It is estimated to affect ~7 million people worldwide, but global surveillance and reporting of infection and disease rates are largely inadequate, due in part to the need for improved diagnostic tools. Mother to child transmission of T. cruzi infection is considered to be “a continuous source of infected newborns, even with complete control of vector and transfusion-mediated transmission”. According to PAHO, an estimated 1.12 million women of childbearing age are infected, and around 9,000 infected babies are born each year, accounting for more than 20% of all new cases in the region. Thus, congenital parasite transmission has implications in terms not only for individual health, but also for global public health. The validation of highly sensitive and specific diagnostics that provide an early access to diagnosis for mothers and newborns will contribute to control the disease impact.
What sort of innovation are you bringing in your project?
The project is designed as an integrated process whereby each of the eight intervention sites will serve as a recruitment center for validation of diagnostic tests, while at the same time will function as a community engagement focus. In the course of the process, we will validate and implement improved algorithms for rapid and reliable diagnosis of pregnant women and newborns. In this regards, validating the use of RDTs for the screening of mothers and taking T. cruzi-LAMP to license gate for its widespread implementation in maternity hospitals represent two major breakthroughs. Diagnosis of infected mothers by ELISAs in endemic regions is subject to delays in results turnaround and often occurs that the mother is unaware of her chronic T. cruzi infection status at the time of delivery. For the newborn, the algorithm to detect congenital infection is lengthy and usually takes several months for serological confirmation with a very high risk of loss to follow-up. Thus, counting with rapid and accurate point-of-care diagnostics will really make the difference.
Role and Responsibility of Each Partner
ISGlobal will coordinate the project being as well responsible for the data management and analysis. EIKEN Chemical Co. will provide its expertise on T. cruzi-LAMP technique and DNA isolation with PURE reagent, while AI Biosciences, Inc. will participate with its low-cost 3D printer-inspired platform to work as a nucleic acids isolation station. CONICET-INGEBI will perform the analytical evaluation to select the best nucleic acids isolation technique to couple to T. cruzi-LAMP, plus it will be the referral laboratory for molecular biology and will transfer the LAMP to the field. Fundacion Mundo Sano, CEADES, University of Nagasaki and CEDIC will be implementing partners, respectively in Argentina, Bolivia and Paraguay. They will enroll and follow all study participants, performing the required serological assays to compare RDTs with ELISAs, as well as they will run the LAMP assays for comparison with currently used congenital Chagas diagnosis algorithm.
Others (including references if necessary)
Besuschio SA et al. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (LAMP) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Negl Trop Dis (2017) 11:e0005779.
Alonso-Padilla J, et al. Strategies to enhance access to diagnosis and treatment for Chagas disease patients in Latin America. Expert Rev Anti Infect Ther (2019) 17(3):145-157.
Lozano D, et al. Use of rapid diagnostic tests (RDTs) for conclusive diagnosis of chronic Chagas disease – a field study in the Bolivian Chaco region. PLoS Negl Trop Dis (2019) 13:e0007877.
Besuschio SA et al. Trypanosoma cruzi loop-mediated isothermal amplification (Trypanosoma cruzi Loopamp) kit for detection of congenital, acute and Chagas disease reactivation. PLoS Negl Trop Dis (2020) 14:e0008402.
Wehrendt DP et al. Development and evaluation of a 3D Printer-based DNA extraction method coupled to loop mediated isothermal amplification (LAMP) for point-of-care diagnosis of congenital Chagas disease in endemic regions. J Mol Diagn (2020) S1525-1578(20)30615-2.
Final Report
1. Project objectives
i) Analytical evaluation of the best DNA isolation methodology for implementing T. cruzi-LAMP as point-of-care (POC) test for timely diagnosis of congenitally acquired T. cruzi infection.
ii) Operationally evaluate T. cruzi-LAMP in hospitals from Chagas disease endemic regions of Argentina, Bolivia and Paraguay.
iii) Validate in the study area the use of rapid diagnostic tests (RDTs) as an alternative to conventional enzyme-linked immunosorbent assays (ELISAs) to detect chronic T. cruzi infections. iv) Increase disease awareness to facilitate the acceptance and adoptability of the technologies under research by the communities and authorities where the project will be developed.
2. Project design
The project design involved two intertwined observational study arms:
(1) Comparison of serological tools (RDTs versus ELISAs) for the detection of chronic T. cruzi infection in mothers. The sample collection and analysis was made in a single visit.
(2) Evaluation of T. cruzi-LAMP as an alternative to the current diagnostic algorithm to detect vertically acquired T. cruzi infection based on microscopy and late serological study of the infants, who were born to those seropositive mothers detected in (1). The collection of samples and their analysis were performed longitudinally at birth, and at months two and nine of the babies.
3. Results, lessons learned
We screened ~7,500 women and found an average prevalence of chronic T. cruzi infection (determined by ELISA) of 9.6%. On a country basis, it was 8.2% in the sites of Argentina, 1.5% in the sites of Paraguay, and 13.6% in Bolivia. The agreement of using RDTs as an alternative to the ELISAs yielded very good specificity (Sp > 0.99), but RDTs sensitivity (Se) was compromised in the study sites of Argentina and Paraguay (Se = 0.49 and Se = 0.62, respectively). In Bolivia, RDTs showed very good capacity to detect true positive infections (Se = 0.88).
Regarding the longitudinal observational study, we managed to complete the follow-up of 545 children born to T. cruzi-infected women. According to the complete gold-standard algorithm, we identified 23 infected children, accounting for a 4.2% vertical transmission rate. Note that such algorithm entails two sub-optimum microscopy-based (micromethod) tests at birth and a few months later, and a subsequent serological study once the infants are nine months old and the risk of false positives from maternally derived anti-T. cruzi antibodies waned. Hence, multiple tests over an almost one year span. Remarkably, the use of T. cruzi-LAMP with liquid whole blood samples collected at birth or two months detected that same number of positives. This is an outstanding result in the shape that the LAMP technology, as a POC molecular amplification tool, can anticipate several months the diagnosis of congenital Chagas disease. Moreover, its agreement to the molecular gold-standard test, the more expensive and complex to operate PCR, was very good (κ = 0.84). Additionally, we obtained a fraction of the newborns samples as dried blood spots (DBS) on filter paper, and compared the LAMP performance using them versus liquid blood, thinking of the scalability of the technology. The agreement was perfect (κ = 1.00).
These promising results encourage us to carry on with the development of the T. cruzi-LAMP prototype. In subsequent calls for proposals, we expect to secure funding to face regulatory agencies demands, license it, perform health economics analysis and further validate its use in other Latin American countries, likely relying on DBS samples.
Investment
Details
Field validation of Trypanosoma cruzi-LAMP: a molecular point-of-care test for the control of congenital Chagas disease




