-
Project IDG2013-105
-
RFP Year2013
-
Awarded Amount$714,500DiseaseMalariaInterventionVaccineDevelopment StagePhase1 Clinical DevelopmentCollaboration PartnersResearch Institute for Microbial Diseases (RIMD), Osaka University , Gulu University, UgandaPublication
-
Tougan T, Ito K, Palacpac NM, Egwang TG, Horii T. Immunogenicity and protection from malaria infection in BK-SE36 vaccinated volunteers in Uganda is not influenced by HLA-DRB1 alleles. Parasitol Int. 2016 Oct;65(5 Pt A):455-8. doi: 10.1016/j.parint.2016.06.012. Epub 2016 Jun 23. PMID: 27343834.
-
Yatsushiro S, Yamamoto T, Yamamura S, Abe K, Obana E, Nogami T, Hayashi T, Sesei T, Oka H, Okello-Onen J, Odongo-Aginya EI, Alai MA, Olia A, Anywar D, Sakurai M, Palacpac NM, Mita T, Horii T, Baba Y, Kataoka M. Application of a cell microarray chip system for accurate, highly sensitive, and rapid diagnosis for malaria in Uganda. Sci Rep. 2016 Jul 22;6:30136. doi: 10.1038/srep30136. PMID: 27445125; PMCID: PMC4995311.
-
Yagi M, Palacpac NM, Ito K, Oishi Y, Itagaki S, Balikagala B, Ntege EH, Yeka A, Kanoi BN, Katuro O, Shirai H, Fukushima W, Hirota Y, Egwang TG, Horii T. Antibody titres and boosting after natural malaria infection in BK-SE36 vaccine responders during a follow-up study in Uganda. Sci Rep. 2016 Oct 5;6:34363. doi: 10.1038/srep34363. PMID: 27703240; PMCID: PMC5050508.
-
Balikagala B, Mita T, Ikeda M, Sakurai M, Yatsushiro S, Takahashi N, Tachibana SI, Auma M, Ntege EH, Ito D, Takashima E, Palacpac NM, Egwang TG, Onen JO, Kataoka M, Kimura E, Horii T, Tsuboi T. Absence of in vivo selection for K13 mutations after artemether-lumefantrine treatment in Uganda. Malar J. 2017 Jan 9;16(1):23. doi: 10.1186/s12936-016-1663-1. PMID: 28068997; PMCID: PMC5223472.
-
Odongo-Aginya EI, Olia A, Luwa KJ, Nagayasu E, Auma AM, Egitat G, Mwesigwa G, Ogino Y, Kimura E, Horii T. Wuchereria bancrofti infection at four primary schools and surrounding communities with no previous blood surveys in northern Uganda: the prevalence after mass drug administrations and a report on suspected non-filarial endemic elephantiasis. Trop Med Health. 2017 Aug 15;45:20. doi: 10.1186/s41182-017-0060-y. Erratum in: Trop Med Health. 2017 Sep 20;45:28. PMID: 28814926; PMCID: PMC5556395.
-
Ikeda M, Kaneko M, Tachibana SI, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, Takahashi N, Yamauchi M, Sekihara M, Hashimoto M, Katuro OT, Olia A, Obwoya PS, Auma MA, Anywar DA, Odongo-Aginya EI, Okello-Onen J, Hirai M, Ohashi J, Palacpac NMQ, Kataoka M, Tsuboi T, Kimura E, Horii T, Mita T. Artemisinin-Resistant Plasmodium falciparum with High Survival Rates, Uganda, 2014-2016. Emerg Infect Dis. 2018 Apr;24(4):718-726. doi: 10.3201/eid2404.170141. PMID: 29553316; PMCID: PMC5875287.
-
Tougan T, Edula JR, Takashima E, Morita M, Shinohara M, Shinohara A, Tsuboi T, Horii T. Molecular Camouflage of Plasmodium falciparum Merozoites by Binding of Host Vitronectin to P47 Fragment of SERA5. Sci Rep. 2018 Mar 22;8(1):5052. doi: 10.1038/s41598-018-23194-9. PMID: 29567995; PMCID: PMC5864917.
-
Ezoe S, Palacpac NMQ, Tetsutani K, Yamamoto K, Okada K, Taira M, Nishida S, Hirata H, Ogata A, Yamada T, Yagi M, Edula JR, Oishi Y, Tougan T, Ishii KJ, Myoui A, Horii T. First-in-human randomised trial and follow-up study of Plasmodium falciparum blood-stage malaria vaccine BK-SE36 with CpG-ODN(K3). Vaccine. 2020 Oct 27;38(46):7246-7257. doi: 10.1016/j.vaccine.2020.09.056. Epub 2020 Oct 2. PMID: 33012605.
-
Tougan T, Edula JR, Morita M, Takashima E, Honma H, Tsuboi T, Horii T. The malaria parasite Plasmodium falciparum in red blood cells selectively takes up serum proteins that affect host pathogenicity. Malar J. 2020 Apr 15;19(1):155. doi: 10.1186/s12936-020-03229-1. PMID: 32295584; PMCID: PMC7161009.
-
Balikagala B, Sakurai-Yatsushiro M, Tachibana SI, Ikeda M, Yamauchi M, Katuro OT, Ntege EH, Sekihara M, Fukuda N, Takahashi N, Yatsushiro S, Mori T, Hirai M, Opio W, Obwoya PS, Anywar DA, Auma MA, Palacpac NMQ, Tsuboi T, Odongo-Aginya EI, Kimura E, Ogwang M, Horii T, Mita T. Recovery and stable persistence of chloroquine sensitivity in Plasmodium falciparum parasites after its discontinued use in Northern Uganda. Malar J. 2020 Feb 18;19(1):76. doi: 10.1186/s12936-020-03157-0. PMID: 32070358; PMCID: PMC7026951.
-
Ntege EH, Arisue N, Ito D, Hasegawa T, Palacpac NMQ, Egwang TG, Horii T, Takashima E, Tsuboi T. Identification of Plasmodium falciparum reticulocyte binding protein homologue 5-interacting protein, PfRipr, as a highly conserved blood-stage malaria vaccine candidate. Vaccine. 2016 Nov 4;34(46):5612-5622. doi: 10.1016/j.vaccine.2016.09.028. Epub 2016 Sep 28. PMID: 27692771.
-
Draper SJ, Angov E, Horii T, Miller LH, Srinivasan P, Theisen M, Biswas S. Recent advances in recombinant protein-based malaria vaccines. Vaccine. 2015 Dec 22;33(52):7433-43. doi: 10.1016/j.vaccine.2015.09.093. Epub 2015 Oct 11. PMID: 26458807; PMCID: PMC4687528.
Introduction and Background of the Project
Med Biotech Laboratories (MBL) and the Research Institute for Microbial Diseases (RIMD), Osaka University have been working together in malaria research for about a decade. Together, they have recently completed a two-stage (6-40 years-old) GCP compliant clinical trial in Lira, Uganda. The study results showed acceptable safety and immunogenicity profiles. In the follow-up study 130-365 days post-second vaccination, protective efficacy against malaria episodes with high parasitemia (>5000 parasites/µL) and fever was 72%. This means that BK-SE36 can afford protection against clinical malaria to vaccinated subjects. However, unlike the 100% response/seroconversion observed in malaria naïve Japanese volunteers, serconversion was low in the malaria endemic area, particularly in older age groups.
Believing that stronger adjuvants can either improve immunogenicity or be capable of eliciting longer-lasting vaccine immune response, RIMD did preclinical trials with the formulation BK-SE36/CpG. CpG oligodeoxynucleotides (CpG ODN) are short, single-stranded synthetic DNA molecules that are being tested as vaccine adjuvants. Following successful preclinical studies, a first-in-human clinical trial (Phase Ia) of BK-SE36/CpG in malaria naïve Japanese adults was initiated with the Medical Center for Translational Research, Osaka University Hospital. Seeking to provide the most suitable and effective vaccine formulation, in the late 2010, the collaboration also widened to include Gulu University under the Program for Strategic Promotion of International Cooperation to Accelerate Innovation in Developing Countries. The present study seeks to select a more “robust” vaccine formulation. Besides the standard evaluation of safety and immunogenicity in phase 1a clinical trials, we want to evaluate the quality of the induced antibody in volunteers and compare these to Phase Ib volunteers as well as to those who have naturally acquired immunity against malaria.How can your partnership (project) address global health challenges?
The partnership seeks to address the global health need of a vaccine for malaria. The absence of a vaccine for malaria underscores the deficiencies in our understanding of the mechanisms and targets of immunity to malaria. Substantial reduction in the disease burden will result from multi-pronged approaches. This is the logic underpinning simultaneous pursuit of various alternatives, as well as different methods to evaluate immune response.
Additional to the antigen, the manner in which vaccine candidates are presented as well as the immunostimulatory effect of the formulation modulates the immune response. It is generally accepted that there is limited immunogenicity and efficacy when vaccine candidates are combined with marketed adjuvants and that lack of suitable adjuvants continue to be a barrier for malaria vaccine development. Although several novel adjuvants are now in licensed vaccines for infectious diseases, access to and information on well-characterized adjuvants remains a challenge. In light of this, the Medical Center for Translational Research, Osaka University Hospital undertook the challenge for an investigator initiated clinical trial for a vaccine formulation that showed significant humoral and cellular responses. In partnership with Gulu University, we aim to understand and obtain evidence of protective immunity by conducting comparative studies to evaluate the quality of immune response (using functional assays) in BK-SE36/CpG volunteers and individuals in malaria endemic areas.What sort of innovation are you bringing in your project?
Reformulating BK-SE36/AHG with CpG as an adjuvant promises enhanced seroconversion. When used for other infectious diseases and other poorly immunogenic malaria vaccine candidates, CpG can improve vaccine immunogenicity, accelerate development of vaccine-induced responses and augment immune response in immunocompromised subjects. An understanding of the mode of action of CpG and the resulting immune responses of the adjuvant/antigen combination will enable us to understand more BK-SE36 and select a robust vaccine not only for the general population but also for vulnerable but difficult target groups, e.g. infants and elderly with weaker immune responses.
Others (including references if necessary)
(1) Palacpac N.M.Q., Ntege E., Yeka A. Balikagala B., Suzuki N., Shirai H., Yagi M., Ito K., Fukushima W., Hirota Y., Nsereko C., Okada T., Kanoi B.N., Tetsutani K., Arisue N., Itagak S., Tougan T., Ishii K.J., Ueda S., Egwang T.G., and Horii T.
Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS ONE. 2013; 8(5): e64073.
(2) Palacpac N.M. Q., Arisue N., Tougan T., Ishii K. J. and Horii T.
Plasmodium falciparum serine repeat antigen 5 (SERA5) as a malaria vaccine candidate. Vaccine. 2011Aug 11;29(35):5837-45.
(3) Horii T, Shirai H, Jie L, Ishii KJ, Palacpac NQ, Tougan T, Hato M, Ohta N, Bobogare A, Arakaki N, Matsumoto Y, Namazue J, Ishikawa T, Ueda S, Takahashi M .
Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol Int. 2 010 Sep;59(3):380-6.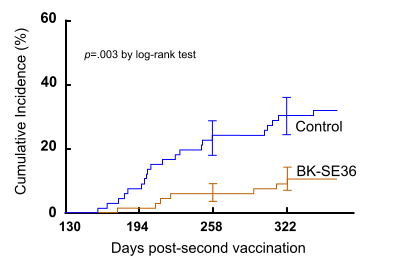
Fig. It is 72% of defense effects in vaccination against BK-SE36 malaria vaccine which we have developed independently in Japan, group judging by the Kaplan Mayer plot.
When viewed simultaneously fever 37.5 ℃ or higher and parasite count ≥ 5000/μL blood, malaria appears.
For comparison with other vaccines, 1.0mL vaccination group sum of 0.5mL of BK-SE36 inoculation group is red line on the figure.
This result shows the protection 72% compared to the control group (p = 0.03).
Final Report
1. Project objective
This is the first-in-human clinical trial (Phase Ia) of BK-SE36/CpG, a blood-stage malaria vaccine formulated with an adjuvant that stimulate innate immunity resulting to high humoral and cellular immune response. Use of the novel adjuvant was evaluated in terms of safety, immunogenicity and the quality of the induced antibodies.
2. Project design
The single-blind, randomized, placebo-controlled, two dose (half- and full-dose) clinical trial was conducted in malaria-naive Japanese adults. Aside from safety and immunogenicity, biological assessments evaluated the immune responses in vaccinees. IgG subclass, protective epitopes and growth inhibitory activities in vaccinees were compared to samples obtained from malaria-exposed population in Africa.
3. Results, lessons learned
In the phase Ia clinical trial in Japan, BK-SE36 mixed with K3 CpG adjuvant was deemed safe and the induced antibody titer was 3-4 fold higher compared to antibody titers from BK-SE36 (with only aluminum hydroxide gel) alone. Also, a level of standardization for key biological assays (IgG subclass, epitope mapping and growth inhibition assays) that can be used to evaluate the efficacy of our malaria vaccine candidate was achieved. We have established serum controls, compared different reagents in terms of their assay sensitivity and specificity that translates to reduced intra-lab variability and greater confidence in assay results. These assays would facilitate evaluation/comparison of results and guide the developmental process of BK-SE36 and BK-SE36/CpG. Physico-chemical studies showed that the structure of SE36 epitopes have characteristics of intrinsically unstructured regions (two regions specified below) and thus a strict tertiary structure may not be required to elicit protective antibodies. This is beneficial for the vaccines’ mass production in contrast to some malaria vaccine candidates that require strict structural conformation in order to elicit protective antibodies. Naturally malaria infected Ugandan sera predominantly reacted with the peptides corresponding to octamer repeat and serine rich sequences, regions previously reported to have limited polymorphism and thus BK-SE36 may also likely overcome challenges with regards to extensive polymorphism. Serum from vaccinees showed reactivity to regions containing polyserine repeats. In pre-clinical studies using mice and squirrel monkeys, reactivity to these regions were observed and challenged infection showed that the vaccinated animals were protected from high parasitemia. In addition, BK-SE36/CpG vaccinated volunteer serum efficiently inhibited parasite growth in vitro. The safety profile, immunogenicity and functionality of the immune response to BK-SE36/CpG warrant further trials in malaria endemic areas.
Investment
Details
Clinical Development of BK-SE36/CpG Malaria Vaccine




