Investment
Case Studies
Development of a Pediatric Formulation
of the Gold Standard Drug for Schistosomiasis
These case studies serve as a bridge between our mission, vision, and product development process by bringing into focus the ultimate goals of the GHIT Fund and all its partners: enabling those in the developing world to break free from the shackles of disease and poverty and pursue the level of prosperity and longevity now common in the industrialized world.
OVERVIEW OF DISEASE IMPACT
In the remote Kenyan village of Muhuru Bay a public health crisis is on the rise. Located on the edge of Lake Victoria, many of the locals rely on the water to make their living through fishing and agriculture irrigated by the lake. The lake water serves as a source for drinking water, washing clothes, and as a playground for school children. Within the lake however, infected snails harbor a parasite which causes a disease called schistosomiasis. The larvae of this parasite are released by these snails and can survive up to 48 hours in the freshwater.
Makena, 8, and her brother Kiano, 3, love to spend their weekends swimming in the lake and playing along the banks of the nearby river while their mother, Winnie, sells produce at the local market. Unknown to the residents of Muhuru Bay, merely coming into contact with the lake’s infested waters puts them at risk of becoming infected with schistosomiasis. And so, one day while swimming in the lake, Makena and Kiano were infected. There weren’t even aware that larvae had penetrated their skin.
For 2 months following infection, the larvae developed into adult worms inside Makena and Kiano’s bodies. The worms move to their internal organs and released eggs. Makena missed school for 2 weeks due to her lingering fever and cough. Prior to becoming sick, Makena was doing well in school but then she began to struggle. Winnie grew more worried when Kiano also fell ill, complaining of painful stomach aches. Winnie had to stay home from work to take care of her children. Across many homes in Muhuru Bay, a similar scene has been unfolding. Infected children stay home for weeks and months. They show signs of impaired cognitive development, stunted growth, malnutrition, and anemia. Family members are forced to stay at home to take care of the children, while others work longer hours to continue to support the family. Adults who work in and around the lake are also being infected with schistosomiasis, showing signs of abdominal pain, fever, fatigue, genital sores, and rashes, among others. It is estimated that over 45 working days are lost due to schistosomiasis infection, per person infected per year1.
Winnie has heard from neighbors about other children with similar symptoms, and the outbreak of a “snail fever.” She rushes Makena and Kiano to the regional health clinic to have them examined. Fortunately, if schistosomiasis infection is suspected, it can be confirmed by a simple urine test. Winnie’s suspicions are confirmed, both Makena and Kiano have moderate cases of schistosomiasis.
Schistosomiasis is treatable with just 1-2 doses of the drug needed to cure the infections. Praziquantel (PZQ), the primary drug used for both prevention and treatment, has been in use since the 1980s. Makena is fortunate -- she will receive PZQ the following week during a school drug administration campaign. The medication will relieve Makena’s pain allowing her to focus on school. Drug administration programs in Kenya, such as the one in Makena’s school, are favored in areas where large number of schistosomiasis infections are suspected. Such programs have proved impactful by gaining 1 year of schooling for every $4 invested in the program2.
 Beyond the cost-effectiveness of the drug therapy itself, schistosomiasis therapy with PZQ also has low
healthcare system costs. There are no reported cases of drug resistance to PZQ, drug administration can be
ably managed by community healthcare workers without specialized medical training, and currently used PZQ
tablets have an excellent storage stability profile. Therefore, as efforts to control schistosomiasis
infections are being considered, access to therapy is the primary consideration.
Beyond the cost-effectiveness of the drug therapy itself, schistosomiasis therapy with PZQ also has low
healthcare system costs. There are no reported cases of drug resistance to PZQ, drug administration can be
ably managed by community healthcare workers without specialized medical training, and currently used PZQ
tablets have an excellent storage stability profile. Therefore, as efforts to control schistosomiasis
infections are being considered, access to therapy is the primary consideration.So the little snails with their invisible parasite larvae have caused considerable impact to the small community of Muhuru Bay. But this disease affects far more than one or two villages. In 2013, it was estimated that 12 million Kenyans were at risk of being infected with schistosomiasis, half of those being children3. Out of all the people at risk, a mere 1.8 million were treated with PZQ. In sub-Saharan Africa, it has been estimated that more than 200,000 deaths per year are due to schistosomiasis4. On a global scale, there were an estimated 240 million people infected with schistosomiasis, with a mere 42 million people of these treated for the disease4. Schistosomiasis also increase the risk that Kiano and Makena will contract other infections. For instance teenage girls and women with female genital schistosomiasis are estimated to have a three times higher likelihood of contracting HIV5.
PZQ USE IN PEDIATRIC POPULATIONS
-
Kiano, age 3, is not as fortunate as his sister. In spite of his diagnosis, he will not receive
treatment for schistosomiasis. Kiano is no exception – there is high prevalence (up to 60%) of
schistosomiasis in children aged less than 4 years old6, however PZQ is typically not
available for use in children of this age group. Exposure to schistosomiasis at such an early
age will leave Kiano vulnerable to increased risk for stunted growth, cognitive impairment, and
other serious health conditions during his later years7.
There are three major hurdles to PZQ dosing in infants and toddlers: (i) the inability for children in this age group to swallow the large-sized commercially available tablets, (ii) high rejection rates due to bitter taste expelled when PZQ tablets are crushed and dissolved in juice and water, and (iii) lower effectiveness (up to 20% lower cure rates) of the crushed tablet suspensions in comparison to PZQ tablets8.
-
EFFECTIVENESS OF MASS DRUG
In Cambodia a long-term drug administration program provided schistosomiasis treatment to the entire population in two endemic areas, and was effective in reducing the prevalence of schistosomiasis from 77% to 0.5%. The reduced prevalence from the drug program, which cost $750,000, resulted in worker productivity gains estimated at $2.7 million9.
ADMINISTRATION PROGRAMS
PEDIATRIC PZQ FORMULATION DEVELOPMENT
-
The Praziquantel Pediatric Consortium, a public-private partnership, was launched to help close
this gap in therapy. With funding from global health funds, such as the GHIT Fund, the
consortium is attempting to develop a pediatric formulation using two approaches: (i) the
development of a new, easier to swallow tablet, and (ii) investigation of a new chemical form of
PZQ (L PZQ), which is potentially less bitter and more effective.
PZQ tablet re-formulation efforts have reduced tablet size to about a quarter of commercially available tablets. This new tablet design potentially allows treatment of infants as young as 3 months old. Taste masking will be a key attribute of the new tablet formulation, and may be achieved either by the use of additives, or the alternate chemical form of PZQ (L-PZQ).
The development of a new pediatric PZQ formulation has potential to significantly impact health outcomes in the pediatric population and ensure that children like Kiano go on to lead long and productive lives. Studies on similar parasitic infections in pre-school age children indicate that infected children had a 20% lower probability of school enrollment, and a 40% reduction in subsequent adult wage earned10. The number of pre-school age children that will benefit from PZQ treatment is not known since these cases often go undiagnosed. However the WHO reported that in 2012 roughly 114 million school age children required preventative PZQ therapy11, reiterating the potentially large impact the new PZQ formulation is likely to provide.
-

PEDIATRIC PZQ – CHALLENGES TO SUCCESSFUL ADOPTION
-
The promise of PZQ therapy available to infants and toddlers is exciting, however significant challenges remain in making pediatric PZQ a reality:
Tablet Stability: Commercially available PZQ tablets are highly stable, and do not need specialized storage requirements. The smaller pediatric formulation under development is designed to rapidly dissolve in the mouth, which may make it more susceptible to moisture and consequently less stable. Specialized storage requirements will not only increase the cost of making the drug available, but may also limit access to largely urban areas with adequate storage infrastructure
Pricing pressure: PZQ tablets are currently available to most governmental health agencies for close to 7 cents per dose, due in part to charitable donations by the drug manufacturer. In order for pediatric PZQ to be effective, it must be delivered in a similar cost-effective manner, facilitated by either governmental purchasing programs or public-private partnership funding
Ability to meet demand: A major challenge to effective control of schistosomiasis remains limited PZQ supply. In 2012, it is estimated that approximately 250 million people required preventative PZQ treatment, however PZQ supply was only sufficient to treat 45 million people. Therefore, for pediatric PZQ to be broadly effective, capital investments will need to be made to ensure its manufacture in sufficient scale to meet the outstanding global -
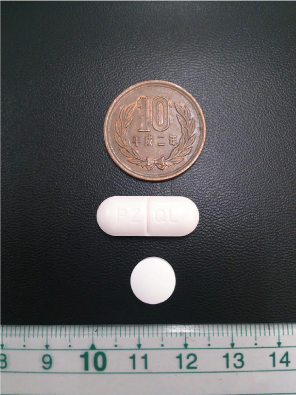 Photo Credit: Astellas Pharma Inc.
Photo Credit: Astellas Pharma Inc.
- 1. Conteh L, Engels T, Molyneux D. Socioeconomic aspects of neglected tropical diseases. The Lancet. 2010;375(9710):239-247. doi:10.1016/S0140-6736(09)61422-7.
- 2. Bitran R1, Martorell B, Escobar L, Munoz R, Glassman A. Controlling and Eliminating Neglected Diseases in Latin America and the Caribbean. Health Affairs. 2009;28(6): 1707-1719. doi:10.1377/hlthaff.28.6.1707.
- 3. PCT databank. World Health Organization Website. http://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/. Accessed September 2014.
- 4. Schistosomiasis. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs115/en/. Updated February 2014. Accessed September 2014.
- 5. Kjetland, EF; Ndhlovu, PD, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593-600. doi: 10.1097/01.aids.0000210614.45212.0a.
- 6. Garba A et al. Schistosomiasis in infants and preschool-aged children: Infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Tropica. 2010;115(3):212-219. doi: 10.1016/j.actatropica.2010.03.005.
- 7. Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends in Parasitology. 2007;23(3):83-86. http://www.ncbi.nlm.nih.gov/pubmed/17241815. Accessed November 10, 2014.
- 8. World Health Organization. Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children. Geneva: World Health Organization; 2011.
- 9. Croce D, Porazzi E, Foglia E., et al. Cost-effectiveness of a successful schistosomiasis control programme in Cambodia (1995-2006). Acta Tropica 2010;113(3):279-84. doi: 10.1016/j.actatropica.2009.11.011.
- 10. Bleakley H. Disease and Development: Evidence from Hookworm Eradication in the American South. The Quarterly Journal of Economics. 2007;122(1);73-117. doi: 10.1162/qjec.121.1.73.
- 11. Schistosomiasis: number of people receiving preventive chemotherapy in 2012. Weekly epidemiological record. 2014;89(2): 21-28. http://www.who.int/wer/2014/wer8902.pdf?ua=1. Accessed November 10, 2014.


